PJ5: Stem cell gene therapy
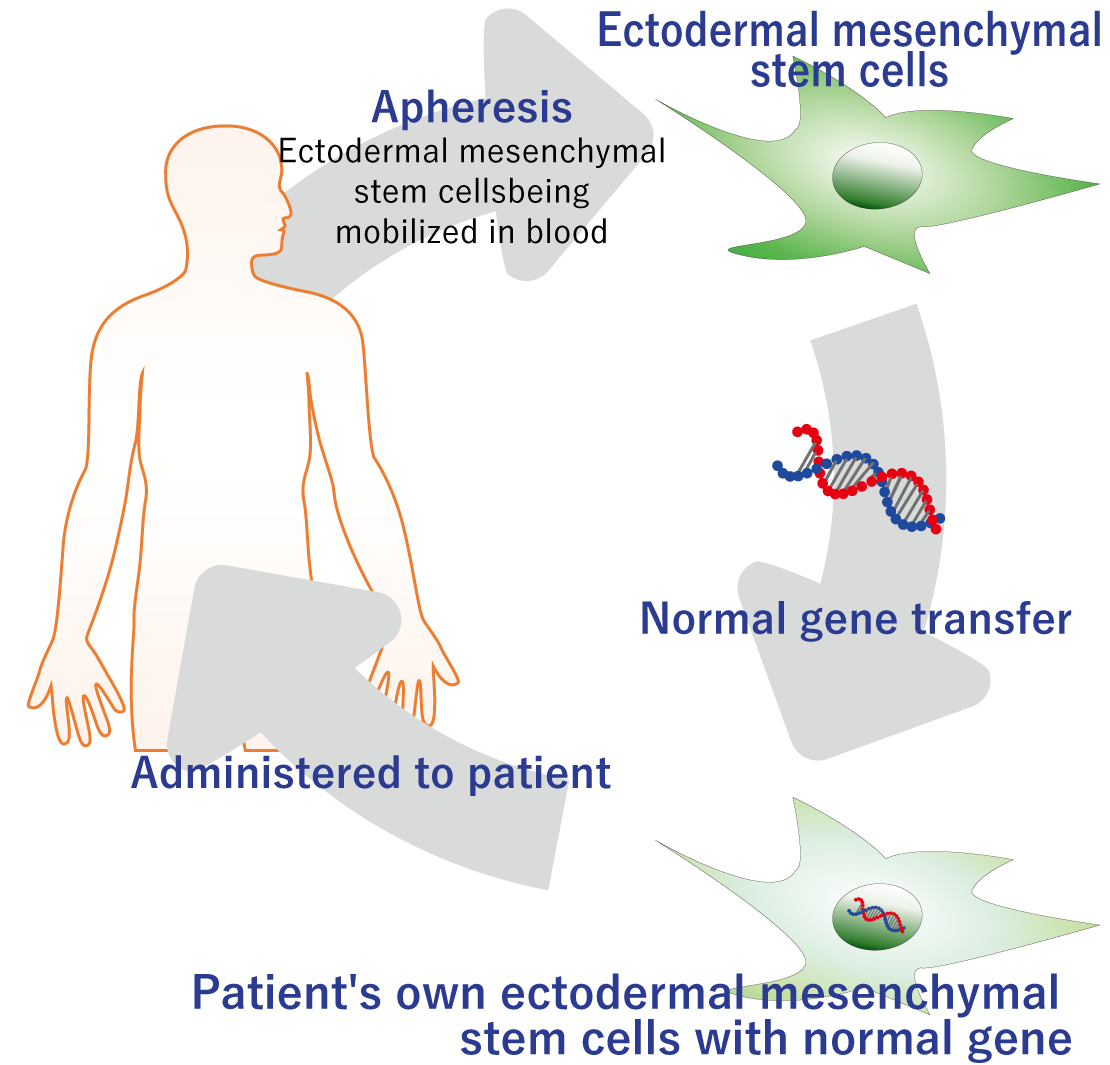
This is a therapeutic technology that enables the use of regeneration-inducing therapy for severe genetic diseases caused by gene deletion, etc. This is a product for radical regeneration-inducing cell therapy utilizing the proprietary stem cell culturing and preparation technology we have developed, with gene editing of the patient’s own stem cells performed outside the body to supply the genes that were deleted or mutated, after which the stem cells are returned to the patient’s body. Gene therapy generally targets stem cells of the affected organ, which means that gene therapy must be performed on different stem cells in the organ for each disease. Normal type VII collagen was genetically transferred into human mesenchymal stem cells and these cells were transplanted into the skin of epidermolysis bullosa model mice. The results demonstrated that human-derived type VII collagen functions normally in the skin of mice. In addition to pluripotency, mesenchymal stem cells have abilities such as immunomodulatory ability, and can have therapeutic effects in a variety of diseases, and thus the use of mesenchymal stem cells in gene therapy is expected to allow the treatment of a variety of genetic diseases.
A normal gene is introduced into mesenchymal stem cells of a genetic disease patient and transplanted to the affected area.
- The therapeutic effect of the therapeutic gene product can be expected.
- The tissue repair promoting action (cell migration action, trophic effect, immunoregulatory action, scar regulation effect, etc.) possessed by ectodermal mesenchymal stem cells can be expected.
- Immune rejection can be expected to be reduced by using self cells.
- Because mesenchymal stem cells have an immune tolerance effect, it can be expected to extend the survival period.