Corporate Information
GREETINGS

In recent times, our society and lifestyles have been changing rapidly, and a variety of fields have begun to shift towards a new age. The bio-venture industry has also witnessed many different changes, and with the introduction of time-limited conditional approval for regenerative medicines in 2014, the SAKIGAKE Designation System in 2015 and the early approval system in 2017, the timeline for obtaining a product approval has been shortened, particularly in the case of products for serious and rare diseases, and there is increasingly greater potential for creating medical products that are effective in treating the diseases patients suffer from.
Against this background, we were established in 2006 as a bio-venture company developing medicine promoting functional tissue regeneration, which enables to treat intractable diseases. Since our foundation, we have consistently worked on the development of “Regeneration-Inducing Medicine™”, which is medicine promoting functional tissue regeneration and enabling the treatment of intractable diseases. At present, we are focusing our business resources on regenerative medicines indicated for a variety of diseases, including epidermolysis bullosa, cerebral infarction and myocardial infarction/cardiomyopathy, autologous cell sampling devices for medical treatment and stem cell gene therapy. “Regeneration-Inducing Medicine™” is a concept with unlimited potential, unlike anything the world has ever seen, and has the potential to produce groundbreaking new drugs that can treat a variety of diseases. At StemRIM, we will continue to take on the challenge of becoming a world-leading bio-venture company by means of “Regeneration-Inducing Medicine™”.
We will continue to push forward with development, so that we can bring smiles to the faces of patients around the world suffering from diseases, including intractable diseases such as epidermolysis bullosa, as soon as we can. We also ask for the continued kind instruction and encouragement of all our stakeholders as we aim to develop our research further.
Masatsune Okajima,
President & CEO
CORPORATE MISSION
Overcoming intractable diseases with
“Regeneration-Inducing Medicine™”.
“Regeneration-Inducing Medicine™” is an innovative new class of medicine that induces functional regeneration of tissues and organs in the patient’s body by maximizing the human body’s innate ability of tissue repairing.
We are pursuing research and development of new drugs based on a novel mechanism of action leading to recruitment of the patients’ own in-vivo stem cells to the damaged tissues for functional tissue regeneration without conventional cell-therapy approaches.
OVERVIEW
| Company name | StemRIM Inc. The origin of the company name of StemRIM is “Stem cell Regeneration-Inducing Medicine”. |
| Established | October 30, 2006 |
| Representative Director | Masatsune Okajima (President & CEO) |
| Number of Employees | 69 (as of July 31, 2025) |
| Capital | 10 million yen (as of July 31, 2025) |
MANAGEMENT
President and CEO
Masatsune Okajima
President and CEO, StemRIM Inc. (Oct. 2023 – Present)
President, StemRIM Inc. (March 2019 – Oct. 2023)
Vice president, Medicinova Inc. (Sep. 2006 – March 2019)
Deputy General Manager, Daiwa Securities SMBC Co., Ltd.(April 2002 – Aug. 2006)
Manager, Daiwa Securities SB Capital Markets Co., Ltd. (currently Daiwa Securities SMBC Co., Ltd.) (April 1999 – March 2002)
Sumitomo Capital Securities Co., Ltd. (Oct. 1996 – April 1999)
Sumitomo Bank, Ltd. (currently Mitsui Sumitomo Bank) (April 1991 – Oct. 1996)

Founder, Chief Scientific Officer
Katsuto Tamai
Director, StemRIM Inc. (Oct. 2022 – Present)
Guest Professor, Endowed course of Regeneration-Inducing Medicine Graduate School of Medicine/ Faculty of Medicine, Osaka University (Oct. 2023 – Present)
Professor, Endowed course of Regeneration-Inducing Medicine Graduate School of Medicine/ Faculty of Medicine, Osaka University (Oct. 2010 – Sep. 2023)
Director, StemRIM Inc. (Feb. 2007 – Aug. 2010)
Associate professor, Department of Stem Cell Therapy Science
The University of Osaka Graduate School of Medicine(May 2003 – Sep. 2009)

External Director
Noriko Sawai
Director, New Business Development
Medical Service Department, NTT Precision Medicine Corporation (Feb. 2024 – Present)
External director, StemRIM Inc. (Oct. 2019 – Present)
Director, Certified Non-Profit Organization “delete C” (Sep. 2019 – Present)
President & CEO, SUN SCHEFFLERA Co., LTD (Oct, 2018 – Present)
Japan Social Innovation and Investment Foundation (Feb.2020- Dec 2023)
DeNA Co. (June 2014 – Nov. 2019)
CSK Venture Capital Co. (April 1995 – May 2014)

External Director
Hirotada Nagai
President, HyakusanSoken KK (July 2022 – Present)
External directors, StemRIM Inc. (Oct. 2020 – Present)
Auditor, Regional Fish Institute, Ltd.
(May 2020 – Present)
Director, PRDM Co., Ltd. (March 2018 – Present)
Director, PorMedTec Co., Ltd. (Dec. 2017 – Present)
Director, Kyoya KK (Dec. 2017 – Present)
Pharmaceuticals and Medical Devices Agency (PMDA)
(Sep. 2012 – July 2014)
Pharmaceutical and Food Safety Bureau of Ministry of Health, Labour and Welfare (April 2001 – Sep. 2017)

External Auditor
Yoji Kudo
Full-time External Auditor, StemRIM Inc. (Oct. 2017 – Present)
Vice President, Global Business Development, Shionogi & Co., Ltd. (2008 – 2016)
Head of Animal Health Division and Corporate Officer, Head of Business Development, Eli Lilly Japan (1985 – 2008)
Uniroyal USA Inc. (1975 – 1985)

External Auditor
Akihiro Mizukami
External Director, Kohjin Bio Co., Ltd. (June 2021 – Present)
External Auditor, KUDO Corporation (Sep. 2020 – Present)
External Auditor, StemRIM Inc. (Oct. 2019 – Present)
Partner in the IPO Support Division of Sanwa Audit Corporation’s Tokyo Marunouchi Office (now Deloitte Tohmatsu) and Head of its Yokohama Office, involved in IPO preparations, audits of listed companies and financial investigations; Representative of Ryohiro Mizukami Certified Public Accountant Office; Director of Rex Advisors Co., Ltd.;

External Audit
Yoichiro Shimada
Joined Sumitomo Bank Ltd. (now Sumitomo Mitsui Banking Corporation) and subsequently served as Head of the Equity Department at Sumitomo Capital Securities Co., Ltd.; Head of the Nagoya Corporate Business Department at Daiwa Securities SMBC Co., Ltd.; Head of the Private Banking Sales Department at Sumitomo Mitsui Banking Corporation; and Auditor of Aoyama Property Networks Co., Ltd. He has served as an Auditor of the Company since October 2019 (Oct. 2019 – Present).

Executive Officer
Takehiko Yamazaki
Executive Vice President & Executive Officer, StemRIM (Aug. 2020 – Aug. 2022)
Vice President & Director, StemRIM (Jan. 2019 – Aug. 2020)
Director, StemRIM (Apr. 2018 – Jan. 2019)
President & Representative Director, StemRIM (Apr. 2010 – Apr. 2018)
Director, StemRIM (Apr. 2007 – Apr. 2010)
Industry–Academia–Government Collaboration Research Fellow, Department of Gene Therapy, Graduate School of Medicine, Osaka University (2003 – 2007)
Assistant Professor, Second Department of Biochemistry, Hirosaki University School of Medicine (2002 – 2003)

BUSINESS MODEL
A biotech company from Osaka University focused on “drug discovery, research and development”
“Regeneration-Inducing Medicine™”, which we have aimed to establish through research and development since our foundation, in collaboration with Osaka University, is medicine based on a completely new and unprecedented mechanism of action, promoting functional regeneration and healing of living tissue that has lost function due to injury or disease.
Unlike conventional regenerative medicine or cell therapy, “Regeneration-Inducing Medicine™” does not require the administration of living cells. Instead, it uses a method where stem cells present in the patient’s own body are activated by administering a substance (the medicine), achieving highly effective regenerative therapy more conveniently and safely. Since the development of “Regeneration-Inducing Medicine™” enables the supply of products achieving rapid regenerative therapy of stable quality in a way that is not feasible using living cell preparations, it has the potential to bring about new regenerative therapies that can be widely adopted.
Stem cells induced by the “Regeneration-Inducing Medicine™” inside the patient’s body are transported inside the body by blood circulation and accumulate specifically in injured tissue. Because the stem cells that have accumulated at the injury site have the ability to differentiate into various types of tissues such as nerves, skin, bone, cartilage, muscle and blood vessels, it is expected that the shared platform of “Regeneration-Inducing Medicine™” can be used to bring a wide range of treatment effects for many of intractable diseases associated with tissue injury, including central nervous system diseases such as cerebral infarction and spinal cord injury, cardiovascular diseases such as myocardial infarction or cardiomyopathy, epithelial diseases such as intractable skin ulcers, and mesenchymal disease such as intractable fractures.
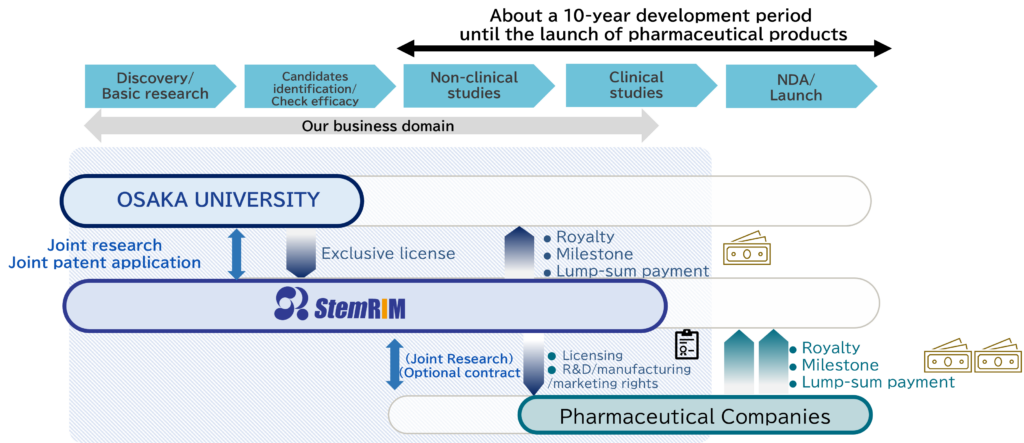
| Type of income | Details |
|---|---|
| Upfront payment | Income obtained as a lump sum when a contract for joint research or licensing is concluded |
| Milestone income | Lump-sum income obtained each time the set target for each development stage (development milestone) is reached, or, after product launch, lump-sum income obtained each time a sales target value (sales milestone) is reached |
| Royalty income | Income obtained after launch as a fixed percentage, specified in advance in a licensing contract, of sales of the product by the pharmaceutical manufacturer with which the licensing contract has been concluded |
| Joint research income | Income obtained as remuneration for conducting joint research using our intellectual property |
LOCATIONS

Head Office
Saito Bio-Incubator 3F, 7-7-15,Saito-Asagi, Ibaraki City, Osaka, Japan

Department of Stem Cell Therapy Science
The University of Osaka Graduate School of Medicine / Department of Stem Cell Gene Therapy Science
The University of Osaka Graduate School of Medicine
The Center of Medical Innovation and
Translational Research, 2-2, Yamadaoka,
Suita City, Osaka, Japan

StemRIM Institute of Regeneration-Inducing Medicine, Osaka University
Techno-Alliance Building, 2-8,
Yamadaoka, Suita-City, Osaka, Japan
CORPORATE GOVERNANCE
Status of Corporate Governance
Our basic corporate governance philosophy
Our corporate mission is to contribute to the well-being and happiness of people globally through developing therapeutic drugs based on new concepts. To pursue and achieve this corporate mission and further increase our corporate value, we recognize that reinforcing and enhancing corporate governance are an important business issue. In a changing business environment, we aim for perpetual development and growth, to continuously maximize our corporate value. To earn the trust of our shareholders and all other stakeholders, we will ensure healthy and efficient management by endeavoring to build an optimal management structure and work to reinforce management auditing functions and ensure highly transparent management through appropriate information disclosure.
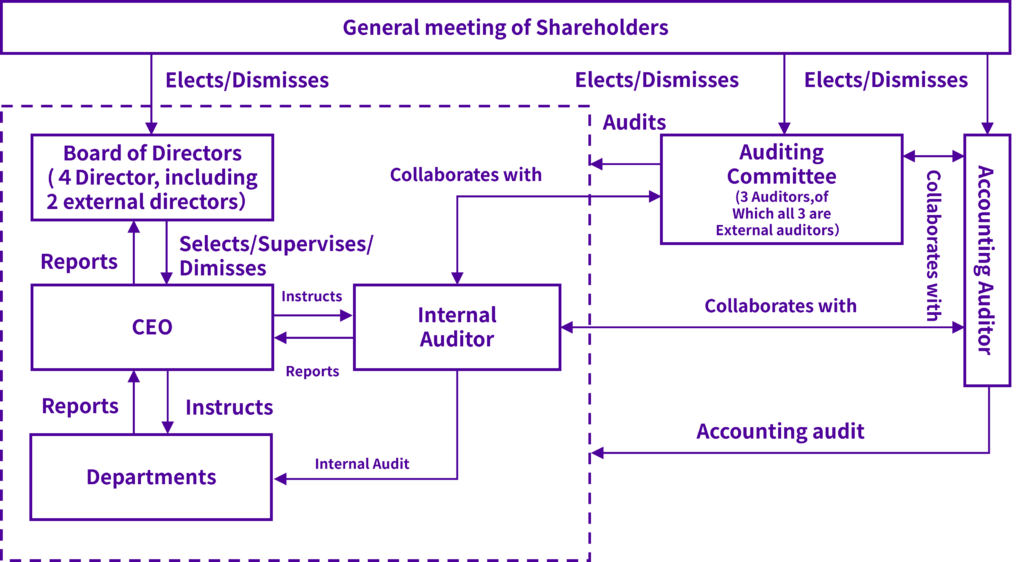
Corporate governance structure
Our board of directors consists of four directors (including two external director). The board of directors holds regular monthly meetings once per month, and extraordinary meetings as necessary, to allow rapid executive decision-making. It decides on the items in our regulations and articles of incorporation and important items relating to management, and audits the status of execution of work by each director. Personnel with detailed knowledge of the pharmaceutical manufacturing industry and corporate management are hired as directors, to enhance the business auditing functions of the board of directors. StemRIM uses an auditing committee system, with an auditing committee consisting of three auditors (of which all three are external auditors), including one permanent auditor. Auditors attend the meetings of the board of directors and give opinions if necessary, and audit the execution of work by directors. In principle, regular auditing committee meetings are held once per month, with extraordinary auditing committee meetings held if necessary, to design auditing plans, to investigate information such as the status and results of audits and share this information between the auditors.
The layout of our corporate governance system is as follows.
Internal control system
The basic philosophy and state of maintenance of our internal control systems are stipulated as shown below, and carried out through the roles of each employee.
(a) A system for ensuring that the execution of the roles of directors and employees conforms to our regulations and articles of incorporation
(b) A system for storing and managing information relating to the execution of the roles of directors
(c) Policies and other systems for managing the risk of loss
(d) A system for ensuring that the roles of directors are executed efficiently
(e) A system for employees that are required by auditors to be designated to assist the roles of the auditors
(f) Items relating to the independence of the employees in (e) above from directors
(g) A system for directors and employees to report to auditors and other systems relating to reporting to auditors
(h) Other systems for ensuring that audits by auditors are performed effectively
(i) A system for excluding organized crime groups
Internal audits and audits by corporate auditors
In order to enable proper management of work and prevent wrongdoing, StemRIM employs a system where two internal auditors are appointed and perform internal audits based on the “internal auditing regulations” that set down the basic rules relating to internal audits. The internal auditors methodically audit the status of work in each department each period and verify that internal rules are complied with and that risks are being prevented. We strive to perform highly effective audits, by regularly checking the status of improvements to matters pointed out by internal audits.
Auditors perform audits effectively, auditing the execution of work by directors based on the auditing plan set down by the auditing committee, for example by attending meetings of the board of directors, investigating company assets and work, and holding regular interviews with the COO.
Auditors, internal auditors and accounting auditors coordinate, for example through exchange of opinions, to increase the effectiveness and efficiency of audits.
Relationships with the external director and external auditors
The Company’s two external directors and three external auditors maintain no personal, business or other interest-based relationships with the Company. Accordingly, we believe they are able to supervise and audit management from a perspective that is independent of the Company’s operations. The role of the external directors is to strengthen the supervisory function of the directors and Board of Directors from an independent perspective. The external auditors liaise closely with the full-time auditors and collect information within the Company as needed. Their role is to strengthen the auditing function of the Board of Auditors. In this manner, the Company has adopted a corporate structure in which the external directors and external auditors function appropriately. As a result, we believe this structure ensures appropriate and efficient decision-making by the Board of Directors.
Although the Company has not set specific standards for the independence of external directors and external auditors, the Company has appointed persons who can perform their duties from an independent and objective standpoint based on their interests and background. In addition, external directors and external auditors cooperate with each other by discussing, reporting, and exchanging information with corporate auditors, internal auditors, and accounting auditors as necessary in their respective supervision or audits.
HISTORY
2006
Oct.
Established a company aiming to develop new drugs based on the discovery of bone marrow multi-potent stem cell mobilization factors identified by Professor Katsuto Tamai of the Graduate School of Medicine, Osaka University.
2007
Apr.
Started collaborative research with Osaka University. We have since continued to create intellectual property from the results of research.
2008
Oct.
Received a research grant from Japan Science & Technology Agency (JST), Industry-Academic Collaborative Seed Innovation Program.
2009
Dec.
Received a research grant from JST, “A-STEP (Adaptable and Seamless Technology Transfer Program through Target-driven R&D)”
2010
Apr.
Transferred our head office to Saito Bio Incubator (Ibaraki City, Osaka Prefecture) and set up a laboratory there.
Concluded a collaborative research contract with Shionogi & Co., Ltd. for research into bone marrow-derived stem cell mobilizing factor.
2011
Nov.
Received a research grant from JST, “A-STEP (Adaptable and Seamless Technology Transfer Program through Target-driven R&D)”
2012
June
Opened Kobe Lab in Port Island, Kobe. Expanded the capacity to perform drug efficacy tests using disease model animals.
2013
July
Expanded the laboratory in Saito Bio Incubator, expanded animal experiment facilities and absorbed the function of Kobe Lab.
2013
Dec.
Received a research grant from JST, “A-STEP (Adaptable and Seamless Technology Transfer Program through Target-driven R&D)”.
2014
Apr.
Adopted as a joint research project of The Center of Medical Innovation and Translational Research (CoMIT), Osaka University Graduate School of Medicine.
2014
May
Received a research grant from the New Energy and Industrial Technology Development Organization (NEDO), Innovation Commercialization Venture Support Project.
2014
Nov.
Concluded a licensing contract with Shionogi & Co., Ltd. for HMGB1 peptides (Redasemtide).
2015
Aug.
An investigator-initiated phase 1 clinical trial of Redasemtide started at Osaka University.
2017
Dec.
An investigator-initiated phase 2 clinical trial of Redasemtide in patients with Dystrophic Epidermolysis Bullosa(DEB) started.
2018
July
Changed our company name to StemRIM Inc.
2019
Apr.
A corporate phase 2 clinical trial of Redasemtide in patients with Acute Ischemic Stroke started by Shionogi & Co., Ltd.
2019
Aug.
Listed on the Tokyo Stock Exchange Mothers
2020
Apr.
An investigator-initiated phase 2 clinical trial of Redasemtide in patients with DEB has been completed.
2020
June
Established a new R&D base, “StemRIM Institute of Regeneration-Inducing Medicine, Osaka University”.
2020
Sep.
The grant project titled ‘Development of Therapeutics for Novel Coronavirus Infection (COVID-19) – Third Call’ has been awarded by AMED (Japan Agency for Medical Research and Development).
2020
Nov.
An investigator-initiated phase 2 clinical trial of Redasemtide in patients with Osteoarthritis of the knees has started at Hirosaki University.
2020
Nov.
An investigator-initiated phase 2 clinical trial of Redasemtide in patients with Chronic Liver Disease has started at Niigata University.
2021
Feb.
A trilateral research agreement has been signed between Shiseido Co., Ltd., Osaka University, for anti-aging research project related to the skin.
2022
July
An additional investigator-initiated phase 2 clinical trial of Redasemtide in patients with DEB has started.
2023
Mar.
A global phase 2b clinical trial of Redasemtide in patients with Acute Ischemic Stroke has started in Japan and North America.
2024
Mar.
An investigator-initiated phase 2 clinical trial of Redasemtide in patients with ischemic cardiomyopathy has started.
2024
Dec.
Selected for the AMED project “FY2024 Project for Fundamental Technology Development toward Industrialization of Regenerative Medicine and Gene Therapy.