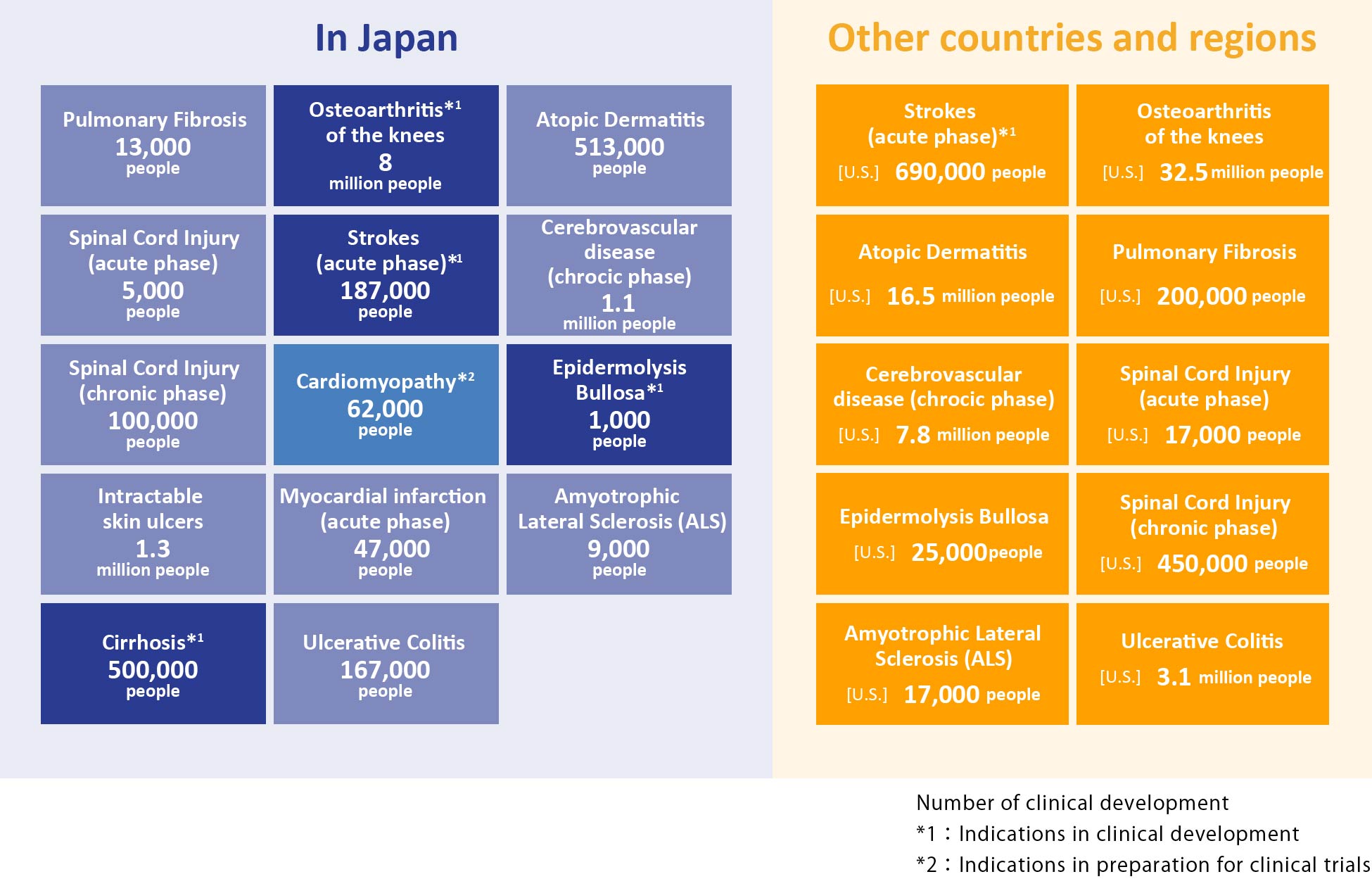
Areas of disease expected as possible indications for “Regeneration-Inducing Medicine™”
Because “Regeneration-Inducing Medicine™” does not act directly on the affected part of the disease, but instead acts on stem cells present in areas such as the bone marrow or blood, it may overcome many issues associated with conventional regenerative medicine/cell therapy, and can target treatment for diseases that have been difficult to treat with conventional therapies. If our “Regeneration-Inducing Medicine™” is put into practice, it is expected to develop a broad range of clinical applications.
Overview of development pipeline
Our research and development pipeline is show below, together with the current state of progress. The pipeline is divided into the following five projects (PJ1 to PJ5). The main target markets for each pipeline are Japan, the US and Europe.
| Project code |
Development candidate | Indication | Investigator | Development stage | Out-license partner |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Research | Pre- clinical |
Phase 1 study |
Phase 2 study |
Phase 3 study |
||||||
| PJ1 | -01 | Redasemtide (HMGB1 cell mobilization domain peptides) |
Epidermolysis bullosa |
Shionogi & Co., Ltd. | Additional P2 Study Ongoing |
Shionogi & Co., Ltd. (S-005151) |
||||
| -02 | Cerebral infarction (acute phase) |
Shionogi & Co., Ltd. | Global P2b Study Ongoing |
|||||||
| -03 | Cardiomyopathy(ischemic cardiomyopathy/dilated cardiomyopathy) | Osaka University | Physician-Initiated P2 Study Ongoing |
|||||||
| -04 | Osteoarthritis of the knee | Hirosaki University | Physician-Initiated P2 study Primary endpoint achieved |
|||||||
| -05 | Chronic liver disease | Niigata University | ||||||||
| PJ2 | -01 | TRIM3(Novel Regeneration-Inducing Peptide for Systemic administration) |
Multiple tissue damage diseases |
In-house (partnership is planned) |
ー | |||||
| -02 | TRIM4(Novel Regeneration-Inducing Peptide for Systemic administration) |
Multiple tissue damage diseases |
In-house (partnership is planned) |
ー | ||||||
| PJ3 | Novel Regeneration-Inducing Peptide for Local administration |
Multiple tissue damage diseases |
In-house (partnership is planned) |
ー | ||||||
| PJ4 | Autologous cell collection device for treatment | Multiple tissue damage diseases |
In-house (partnership is planned) |
ND | ー | |||||
| PJ5 | Stem cell gene therapy | Epidermolysis bullosa |
In-house (partnership is planned) |
Phase 1,2 study | non | ー | ||||
In the case of PJ1 -01, the number of target patients with epidermolysis bullosa dystrophica, is around 400 in Japan, and therefore it is not feasible to plan a large-scale phase 3 study. In addition, epidermolysis bullosa dystrophica is a rare intractable disease, and there is currently no effective therapy. Accordingly, we expect to apply for a marketing approval for the drug based on the results of the additional phase 2 study.
In the case of PJ4, we are currently coordinating development plans, and do not currently expect to conduct studies after phase I, but as no final decision has been made, this is marked ND.
