Pipeline
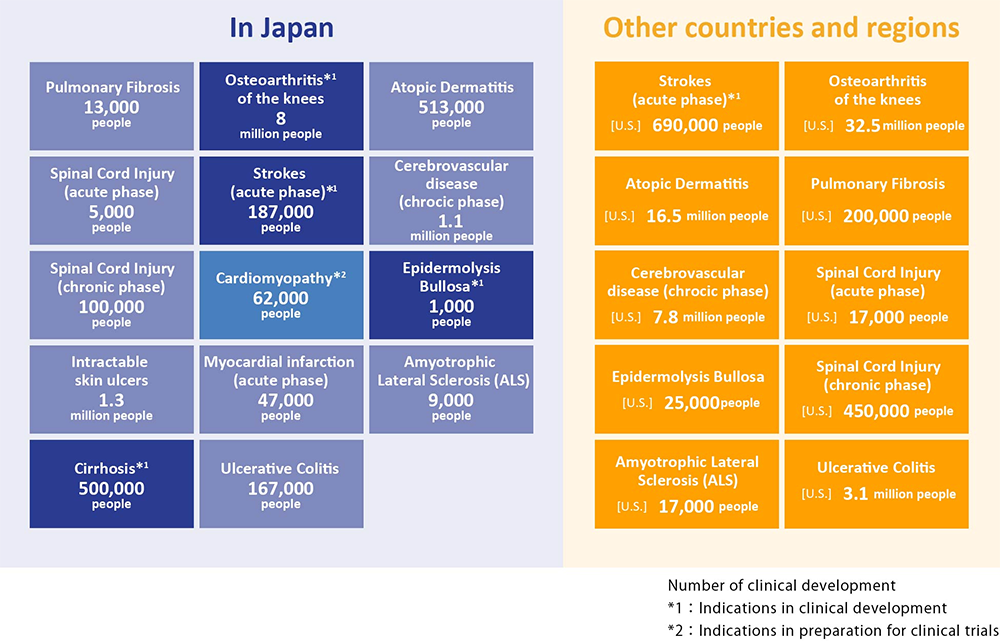
Areas of disease expected as possible indications for “Regeneration-Inducing Medicine™”
Because “Regeneration-Inducing Medicine™” does not act directly on the affected part of the disease, but instead acts on stem cells present in areas such as the bone marrow or blood, it may overcome many issues associated with conventional regenerative medicine/cell therapy, and can target treatment for diseases that have been difficult to treat with conventional therapies. If our “Regeneration-Inducing Medicine™” is put into practice, it is expected to develop a broad range of clinical applications.
Overview of development pipeline
Our research and development pipeline is show below, together with the current state of progress. The pipeline is divided into the following five projects . The main target markets for each pipeline are Japan, the US and Europe.
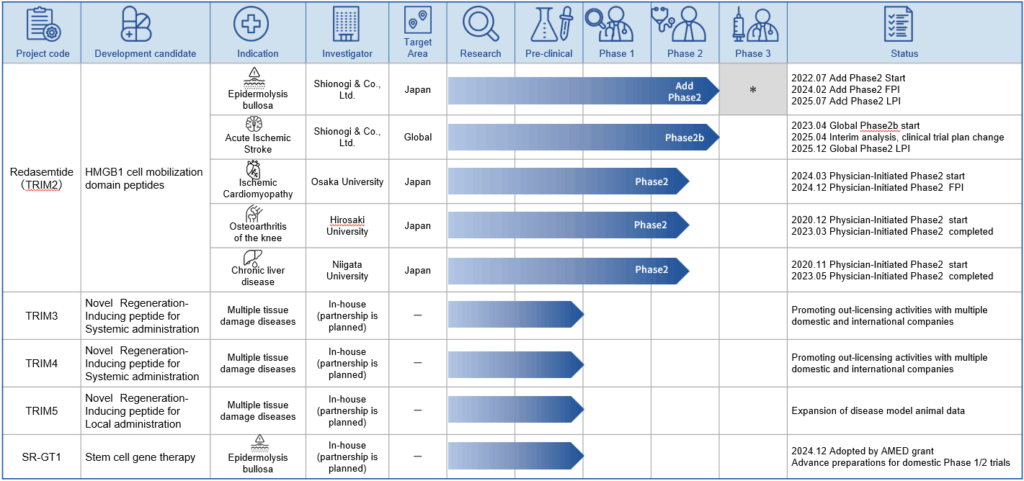
In the case of epidermolysis bullosa, the number of target patients with epidermolysis bullosa dystrophica, is around 400 in Japan, and therefore it is not feasible to plan a large-scale phase 3 study. In addition, epidermolysis bullosa dystrophica is a rare intractable disease, and there is currently no effective therapy. Accordingly, we expect to apply for a marketing approval for the drug based on the results of the additional phase 2 study.
TRIM2: Redasemtide(HMGB1 peptide)
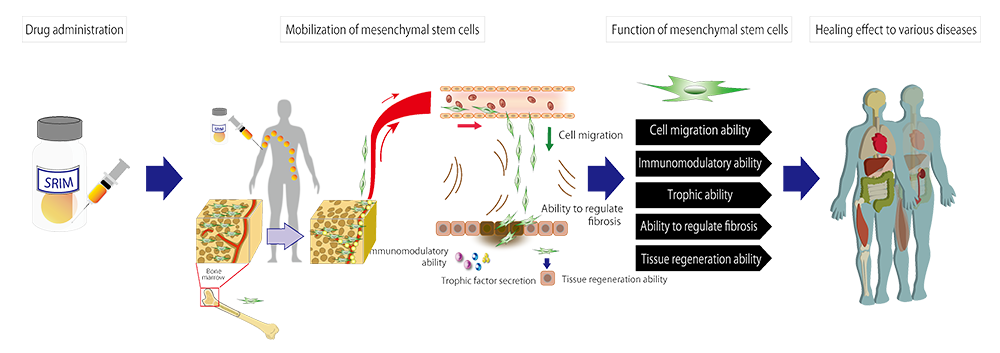
TRIM2 is a peptide drug created from an active domain of a human-endogenous protein HMGB1. By intravenous injection, it mobilizes the patient’s own mesenchymal stem cells in the bone marrow to enter the blood stream and accumulate at the damaged tissue. This promotes the regeneration of the damaged tissues. TRIM2 is expected to be effective in a broad range of diseases because mesenchymal stem cells trigger various functions such as immunomodulation, trophic effects, fibrosis attenuation, and tissue regeneration. Therapeutic effects have been confirmed in the following animal disease model studies:
- Epidermolysis bullosa
- Cerebral infarction
- Myocardial infarction/cardiomyopathy
- Chronic Liver Disease
- Knee osteoarthritis
Because the active ingredient of the drug is a chemically synthesized peptide, it can circumvent the challenges of the regenerative medicine and cell therapies such as manufacture, transportation, storage, and administration. And it can treat diseases that are difficult to treat with conventional medicines.
TRIM3/4
Since the discovery of the peptide that activates the bone marrow mesenchymal stem cells to regenerate the damaged tissue, we have focused our research on the innate mesenchymal stem cells that exist in our bodies, the ones residing in the bone marrow, the ones circulating in the bloodstream, and the ones existing in the damaged tissues. Based on the findings from our research, we have created peptide design and screening methods for activating the mesenchymal stem cells. Using these methods, we have accelerated our research and development of our next-generation Regeneration-Inducing Medicine™.
As a result, we have succeeded in developing novel regeneration-inducing peptides, TRIM3 and TRIM4, which mobilize the mesenchymal stem cells that reside in the bone marrow of our bodies. TRIM3 and TRIM4 are expected to be effective in a wide range of diseases involving tissue damage.
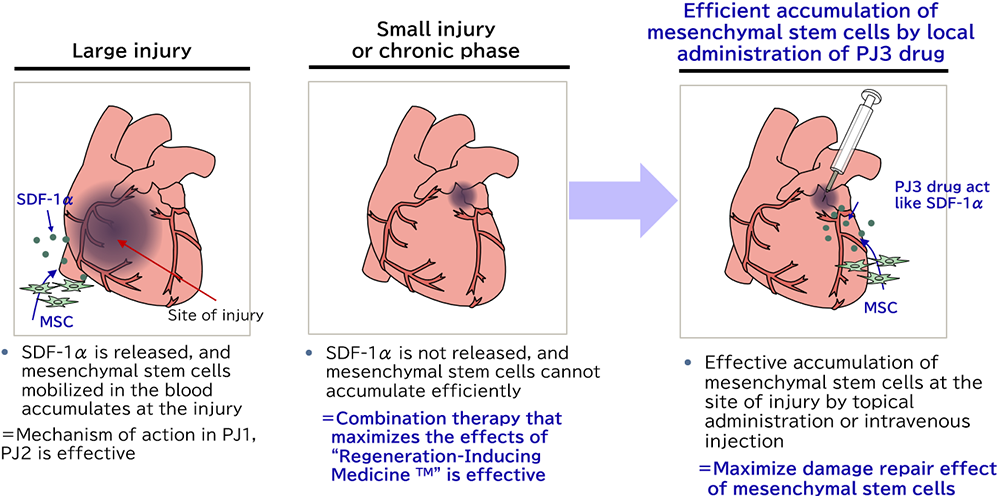
TRIM5
TRIM5 is a molecule, which guides innate mesenchymal stem cells to the damaged tissue to promote tissue repair. In the disease with a small lesion, the ischemic area is small, resulting in a smaller amount of secreted SDF-1α, which is a chemokine increased under hypoxic conditions. Insufficient SDF-1α can cause the failure of the mesenchymal stem cells to migrate to the damaged tissue. By injecting TRIM5 around the lesion, the mesenchymal stem cells circulating in the bloodstream are attracted to the tissue that needs to be treated.
SR-GT1 (Stem Cell Gene Therapy)
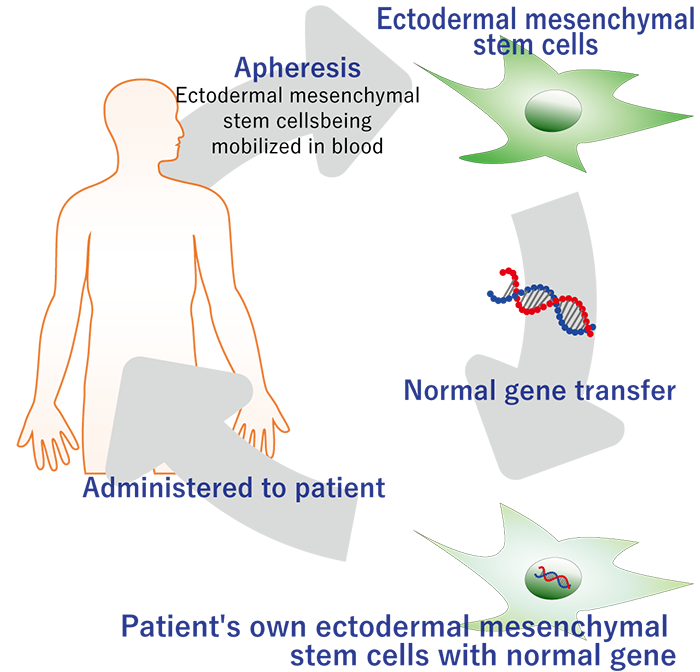
SR-GT1 is a gene therapy that applies our accumulated knowledge of Regeneration-Inducing Medicine™ to cure a severe genetic disease, epidermolysis bullosa. Leveraging our stem cell collection and culture technologies, we deliver the functional gene to the patient’s own stem cells outside of the patient’s body and return the cells to the patient.
In SR-GT1, we harvest mesenchymal stem cells from a blister of the patient and transduce them with a lentiviral vector that delivers correct collagen type VII gene. We then expand and administer them into the blisters of the patient. Thereby, the functional collagen type VII protein is deposited in the patient’s skin, and its therapeutic effect will be sustained. Because we use the patient’s own cells, SR-GT1 is expected to be very safe. In addition, intrablister administration of the gene-corrected cells makes the treatment very efficient.
Method
A normal gene is introduced into mesenchymal stem cells of a genetic disease patient and transplanted to the affected area.
Expected effect
- The therapeutic effect of the therapeutic gene product can be expected.
- The tissue repair promoting action (cell migration action, trophic effect, immunoregulatory action, scar regulation effect, etc.) possessed by ectodermal mesenchymal stem cells can be expected.
- Immune rejection can be expected to be reduced by using self cells.
- Because mesenchymal stem cells have an immune tolerance effect, it can be expected to extend the survival period.